
Raoult's Law For Non Volatile Solute. Raoults law for volatile solutes. The presence of a solute leads to a colligative property called the lowering of the vapor pressure of the solution when compared to the vapor pressure of the pure solvent. If solute is non volatile solid or liquid the vapour pressure of solution is equal to partial vapour. This is solution 22(raoult's law for volatile and non volatile solute) by anindya joardar on vimeo, the home for high quality videos and the people… It goes on to explain how the resulting lowering of vapour pressure affects the boiling. Raoult's law indicates the behavior of solvent in a solution that is in equilibrium with its vapor pressure. Raoult's law states that in a solution, the vapour pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the total vapour pressure p of a solution containing two components a and b is. This article describes the basis of raoult's law and this blend puts us comfortably in the middle of the of typical automotive lpg vapor pressure specifications. Consider a pure liquid in a beaker is covered with a jar. Raoult's law (/ˈrɑːuːlz/ law) is a law of physical chemistry, with implications in thermodynamics. State raoult's law for the solution containing volatile components.
In 1887, raoult put forward a law which gives relationship b/w the mole fraction of solute(volatile as well as non volatiile)and its vappour pressure in solution is called raoult law. It states that the partial vapor pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure component multiplied by its mole fraction in the mixture. State raoult's law for the solution containing volatile components. Consider a pure liquid in a beaker is covered with a jar. Raoult's law (/ˈrɑːuːlz/ law) is a law of physical chemistry, with implications in thermodynamics. This is the definition of raoult's law in chemistry and a description of when to use it and exceptions. However, there are certain limitations when henry's law constant is a proportionality constant and is dependent on the type of solvent, solute, and the temperature. Raoult's law states that in a solution, the vapour pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the total vapour pressure p of a solution containing two components a and b is. The relative lowering of vapour pressure for a solution is equal to the mole fraction of solute when solvent alone is volatile. It goes on to explain how the resulting lowering of vapour pressure affects the boiling.
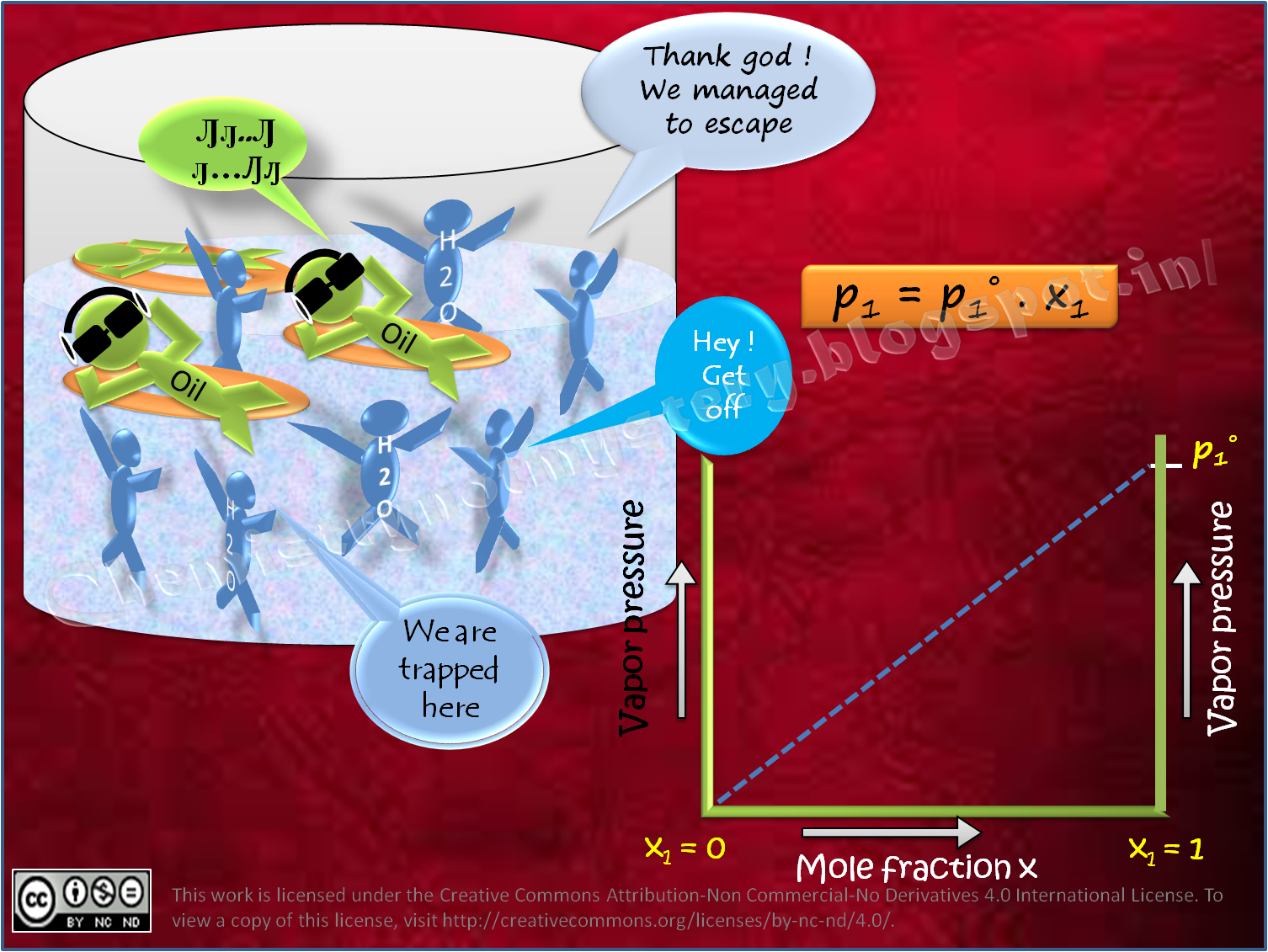
Raoult's law assumes the physical properties of the components of a chemical solution are identical.
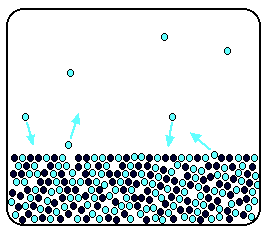
The effect of nonvolatile solutes on vapor pressure. <br>between raoult's law and henry's law ? It states that the partial vapor pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure component multiplied by its mole fraction in the mixture. Raoult's law (/ˈrɑːuːlz/ law) is a law of physical chemistry, with implications in thermodynamics. Number of moles of the solute. The pressure at which vapor is formed above a solid or liquid at a particular temperature is called the vapor pressure. Raoults law for volatile solutes. The presence of a solute leads to a colligative property called the lowering of the vapor pressure of the solution when compared to the vapor pressure of the pure solvent. Therefore, for a particular gas. Raoult's law states that in a solution, the vapour pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the total vapour pressure p of a solution containing two components a and b is.
Raoult's law regarding volatile solutes. State raoult's law for the solution containing volatile components. The relative lowering of vapour pressure for a solution is equal to the mole fraction of solute when solvent alone is volatile. Raoults law for volatile solutes. Raoult's law indicates the behavior of solvent in a solution that is in equilibrium with its vapor pressure. However, there are certain limitations when henry's law constant is a proportionality constant and is dependent on the type of solvent, solute, and the temperature. Mathematically, raoult's law for a single component in an ideal solution is stated as. This is solution 22(raoult's law for volatile and non volatile solute) by anindya joardar on vimeo, the home for high quality videos and the people… For dilute the molecules of the solute are too far apart to interact.

Number of moles of the solute.
Determining vapor pressure in relation to solutes in solutions. X= mole fraction of solvent ; Raoult's law only works for ideal solutions. Number of moles of the solute. In elementary applications, raoult's law is generally valid when the liquid. In 1887, raoult put forward a law which gives relationship b/w the mole fraction of solute(volatile as well as non volatiile)and its vappour pressure in solution is called raoult law. The effect of nonvolatile solutes on vapor pressure. For dilute the molecules of the solute are too far apart to interact. Raoults law for volatile solutes. If solute is non volatile solid or liquid the vapour pressure of solution is equal to partial vapour. The presence of a solute leads to a colligative property called the lowering of the vapor pressure of the solution when compared to the vapor pressure of the pure solvent. P= vapour pressure of an ideal solution ;
Raoult's law is a phenomenological law that assumes ideal behavior based on the simple the solute also shows a linear limiting law, but with a different coefficient. State raoult's law for the solution containing volatile components. X= mole fraction of solvent ; Raoult's law only works for ideal solutions. The effect of nonvolatile solutes on vapor pressure. Consider a pure liquid in a beaker is covered with a jar. The vapour pressure of any component at given temperature is the product of mole fraction of the component in solution with vapour for a binary mixture of two volatile component a and b with mole fraction xa and xb with pure component vapour pressure respectively. Raoult's law for volatile liquids. Raoult's law with example problem. The presence of a solute leads to a colligative property called the lowering of the vapor pressure of the solution when compared to the vapor pressure of the pure solvent.
X= mole fraction of solvent ;
Raoult's law with example problem. For dilute the molecules of the solute are too far apart to interact. Number of moles of the solute. Raoult's law (/ˈrɑːuːlz/ law) is a law of physical chemistry, with implications in thermodynamics. Mathematically, raoult's law for a single component in an ideal solution is stated as. The vapour pressure of any component at given temperature is the product of mole fraction of the component in solution with vapour for a binary mixture of two volatile component a and b with mole fraction xa and xb with pure component vapour pressure respectively. In elementary applications, raoult's law is generally valid when the liquid. Raoult's law indicates the behavior of solvent in a solution that is in equilibrium with its vapor pressure. Consider a pure liquid in a beaker is covered with a jar. Raoults law for volatile solutes. It goes on to explain how the resulting lowering of vapour pressure affects the boiling.
This is the definition of raoult's law in chemistry and a description of when to use it and exceptions raoult's law. State raoult's law for the solution containing volatile components.
 Source: edge.uacdn.net
Source: edge.uacdn.net The vapour pressure of any component at given temperature is the product of mole fraction of the component in solution with vapour for a binary mixture of two volatile component a and b with mole fraction xa and xb with pure component vapour pressure respectively.
 Source: i.ytimg.com
Source: i.ytimg.com Raoult's law only works for ideal solutions.
 Source: i.ytimg.com
Source: i.ytimg.com Raoult's law with example problem.
 Source: i.ytimg.com
Source: i.ytimg.com However, there are certain limitations when henry's law constant is a proportionality constant and is dependent on the type of solvent, solute, and the temperature.
Get acquainted with the concepts of raoults law with the help of study material for iit jee by askiitians.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com This is solution 22(raoult's law for volatile and non volatile solute) by anindya joardar on vimeo, the home for high quality videos and the people…
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com The relative lowering of vapour pressure for a solution is equal to the mole fraction of solute when solvent alone is volatile.
 Source: image.slideserve.com
Source: image.slideserve.com Raoult's law assumes the physical properties of the components of a chemical solution are identical.
 Source: chem.libretexts.org
Source: chem.libretexts.org The presence of a solute leads to a colligative property called the lowering of the vapor pressure of the solution when compared to the vapor pressure of the pure solvent.
 Source: i.ytimg.com
Source: i.ytimg.com Raoult's law for volatile liquids.
 Source: i.ytimg.com
Source: i.ytimg.com Raoult's law (/ˈrɑːuːlz/ law) is a law of physical chemistry, with implications in thermodynamics.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net A french chemist, francois marte raoult gave the relationship between partial pressure and mole fraction of two components.
 Source: i.ytimg.com
Source: i.ytimg.com Raoult's law gives a method of estimating the composition and pressure of the vapour above a liquid mixture.
 Source: i.ytimg.com
Source: i.ytimg.com Raoult's law (/ˈrɑːuːlz/ law) is a law of physical chemistry, with implications in thermodynamics.
 Source: blogmedia.testbook.com
Source: blogmedia.testbook.com In elementary applications, raoult's law is generally valid when the liquid.
 Source: i.ytimg.com
Source: i.ytimg.com Therefore, for a particular gas.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com Raoult's law regarding volatile solutes.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com This is the definition of raoult's law in chemistry and a description of when to use it and exceptions.
 Source: edge.uacdn.net
Source: edge.uacdn.net The presence of a solute leads to a colligative property called the lowering of the vapor pressure of the solution when compared to the vapor pressure of the pure solvent.
 Source: i.ytimg.com
Source: i.ytimg.com In elementary applications, raoult's law is generally valid when the liquid.
 Source: i.ytimg.com
Source: i.ytimg.com Raoult's law regarding volatile solutes.
 Source: i.ytimg.com
Source: i.ytimg.com It goes on to explain how the resulting lowering of vapour pressure affects the boiling.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com It goes on to explain how the resulting lowering of vapour pressure affects the boiling.
 Source: cdn3.edurev.in
Source: cdn3.edurev.in This is the definition of raoult's law in chemistry and a description of when to use it and exceptions.
However, there are certain limitations when henry's law constant is a proportionality constant and is dependent on the type of solvent, solute, and the temperature.
 Source: xpectare.info
Source: xpectare.info The pressure at which vapor is formed above a solid or liquid at a particular temperature is called the vapor pressure.
 Source: www.chemguide.co.uk
Source: www.chemguide.co.uk Raoult's law with example problem.
 Source: classconnection.s3.amazonaws.com
Source: classconnection.s3.amazonaws.com In such ideal solution the vapour pressure of the volatile solvent is equal to the product.
 Source: image.slidesharecdn.com
Source: image.slidesharecdn.com Raoults law for volatile solutes.
 Source: www.chemguide.co.uk
Source: www.chemguide.co.uk Raoult's law indicates the behavior of solvent in a solution that is in equilibrium with its vapor pressure.
 Source: xpectare.info
Source: xpectare.info This article describes the basis of raoult's law and this blend puts us comfortably in the middle of the of typical automotive lpg vapor pressure specifications.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com Raoult's law for volatile liquids.
 Source: i.ytimg.com
Source: i.ytimg.com The effect of nonvolatile solutes on vapor pressure.
 Source: i.ytimg.com
Source: i.ytimg.com However, there are certain limitations when henry's law constant is a proportionality constant and is dependent on the type of solvent, solute, and the temperature.
Posting Komentar untuk "Raoult's Law For Non Volatile Solute"